Rufomycins (Not Ilamycins)
Overview
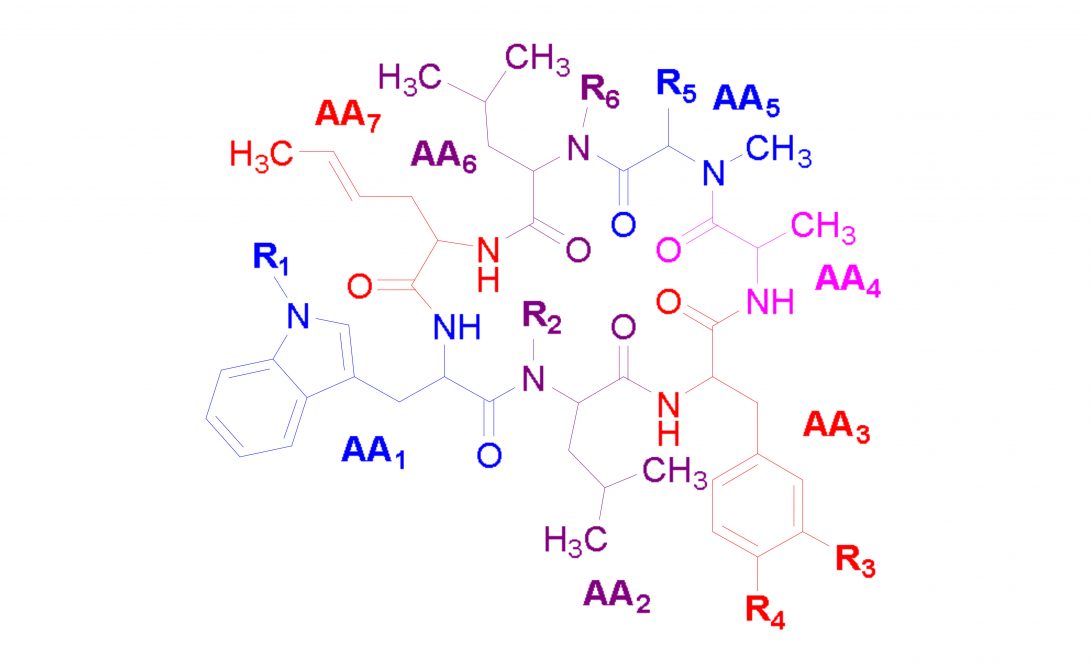
Nomenclature of the Rufomycin Family of Cycloheptapeptides | A Path to Consistency
The rufomycins are a class of cyclic heptapetides that are found in certain Streptomyces species and are receiving major attention as potential anti-tuberculosis drug leads. A significant degree of confusion exists with regard to their names and, consequentially, their exact chemical structures. Notably:
- The structures of the rufomycins are sufficiently complex to cause major confusion (and error) when preparing the typical 2D chemical drawings, especially when using various orientations of the molecules on the paper/drawing plane
- This is exacerbated by the
- numerous chiral centers, beyond the D-/L-nature of the amino acids, as well as
- chemically semi-labile functional groups (e.g., a hemi-aminal ring)
- the formation of various meta-stable conformers at room and physiological temperatures
Rufomycins, not Ilamycins
The naming confusion applies particularly with regard to the use of “ilamycins” as synonymous name for the rufomycins. The use of “ilamycins” adds unnecessary ambiguity. The following 2021 publications seeks to clarify the situation and provides a full historic, structural, and analytical account of the rufomycins, including why the rufomycin name takes clear precedence over the ilamycin name.
B Zhou, P Achanta, G Shetye, SN Chen, H Lee, YY Jin, J Cheng, MJ Lee, JW Suh, SH Cho, S Franzblau, G Pauli, J McAlpine
Rufomycins or Ilamycins: Naming Clarifications and Definitive Structural Assignments
Journal of Natural Products 84, 2644-2663 (2021) dx.doi.org/10.1021/acs.jnatprod.1c00198 [Note: this is an Open Access article]
This Page Undergoes Constant Update
This site is being updated continuously to keep pace with the literature. Particularly, we seek to include the latest members of this class of compounds along with their consolidated rufomycin name and number.
The Rufomycins Reference Table and "Ilamycin" Synopsis
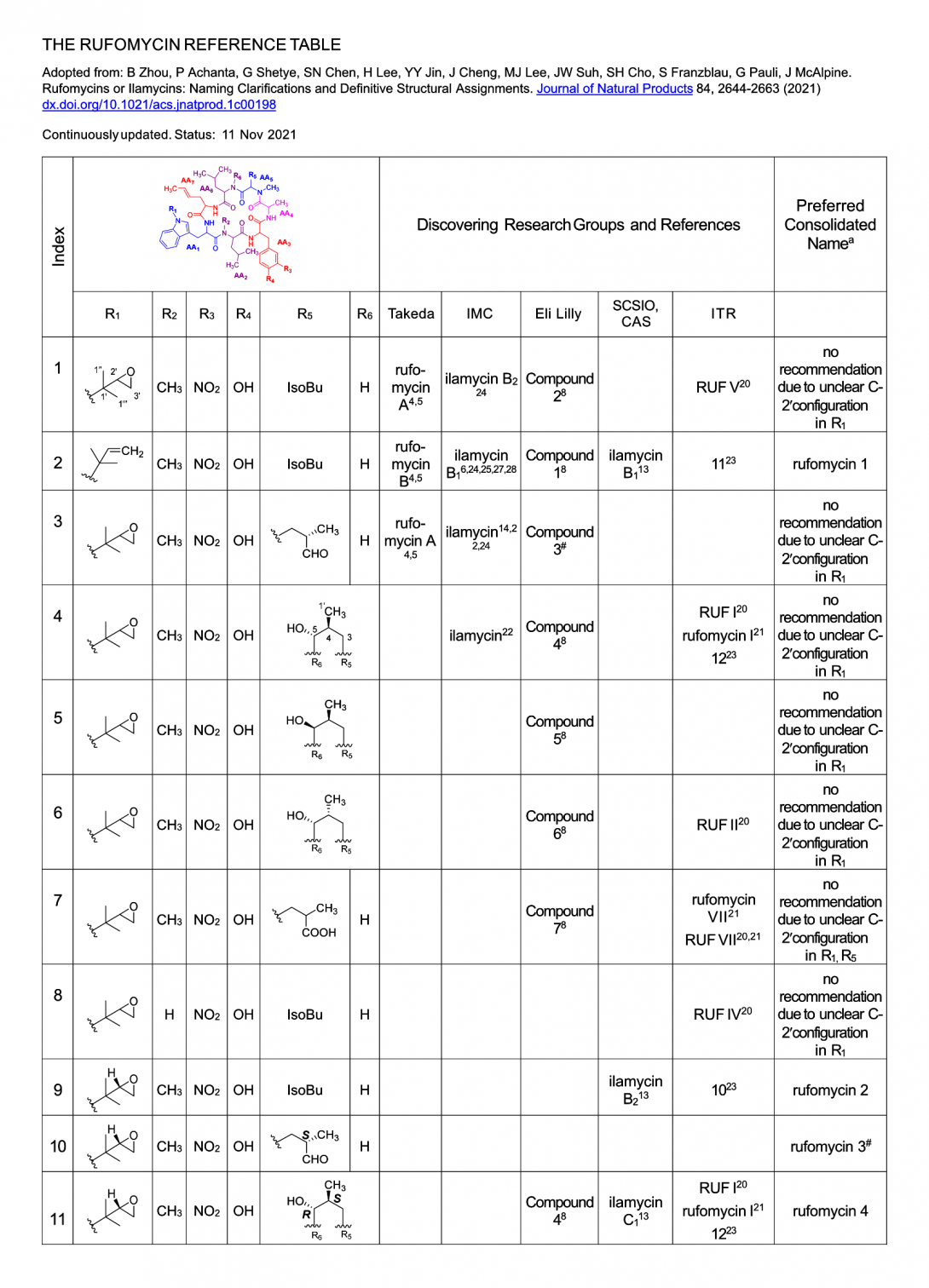
Table of Rufomycin Names and Structures | Synopsis with “Ilamycin” Names
Based on Table 1 in Zhou et al., Journal of Natural Products 84, 2644-2663 (2021) dx.doi.org/10.1021/acs.jnatprod.1c00198] The following materials compile the structures, a synopsis of published names with references, and preferred consolidated names of the rufomycin family of cyclopeptides, including compounds previously named as “ilamycins”.
Important Footnote Information
[footnote a in original table] The numbering used has been derived from that of the Eli Lilly scientists who were the first to discover and structurally elucidate significant numbers of this family of heptapeptides. With the exception of rufomycin 1, an olefin, which was the first of the family to be structurally characterized, the numbers up to 20 have been reserved for epoxides on the isoprenyl group of AA1, numbers 21-40 for the corresponding olefins, 41-50 for other oxidations of this group and above 50 for variants involving changes in that precede the assembly via the NRPS. The semisynthetics (rufomycinSS) have been numbered by us and according to the Eli Lilly group the only groups that have published on semisynthetics.
[footnote # in original table] While these compounds have not been isolated, nor is any spectroscopic data for them available, their transitory existence is not in doubt. Rufomycin 12 is probably not a natural product but rather an artifact of the isolation as suggested. The same may be the case for rufomycin 30.